Thiamethoxam
| PubChem CID | 5821911 |
| Molecular Formula | C8H10ClN5O3S |
| Structure |
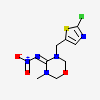
|
| Synonyms |
Thiamethoxam
Actara 153719-23-4 Thiamethoxam [ISO] (E)-thiamethoxam Cruiser Diacloden C8H10CLN5O3S 3-((2-Chloro-5-thiazolyl)methyl)tetrahydro-5-methyl-N-nitro-4H-1,3,5-oxadiazin-4-imine 747IC8B487 Actara 2GR Actara 25WG (4E)-3-[(2-chloro-1,3-thiazol-5-yl)methyl]-5-methyl-N-nitro-1,3,5-oxadiazinan-4-imine 4H-1,3,5-Oxadiazin-4-imine, 3-((2-chloro-5-thiazolyl)methyl)tetrahydro-5-methyl-N-nitro- Adage 5FS n-(3-((2-chlorothiazol-5-yl)methyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene)nitramide 4H-1,3,5-Oxadiazin-4-imine, 3-[(2-chloro-5-thiazolyl)methyl]tetrahydro-5-methyl-N-nitro- CGA 293343 CCRIS 9026 UNII-747IC8B487 HSDB 7938 3-(2-Chloro-5-thiazolylmethyl)tetrahydro-5-methyl-N-nitro-4H-1,3,5-oxadiazin-4-imine Thiamethoxam 10 microg/mL in Acetonitrile Thiamethoxam 100 microg/mL in Acetonitrile THIAMETHOXAM [MI] EC 428-650-4 SCHEMBL22916 CHEBI:39186 (E)-N-(3-((2-chlorothiazol-5-yl)methyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene)nitramide 3-(2-Chloro-thiazol-5-ylmethyl)-5-methyl(1,3,5)oxadiazinan-4-ylidene-N-nitroamine HY-B0833 AKOS015963217 AKOS040742726 AC-8802 4H-1,3,5-Oxadiazin-4-imine, 3-(2-chloro-5-thiazolyl)methyltetrahydro-5-methyl-N-nitro- AS-14085 1ST20350-1000B CS-0012847 Thiamethoxam Solution in Acetone, 1000?g/mL C18513 Thiamethoxam, PESTANAL(R), analytical standard A809460 Q1040678 (EZ)-3-(2-CHLORO-1,3-THIAZOL-5-YLMETHYL)-5-METHYL-1,3,5-OXADIAZINAN-4-YLIDENE(NITRO)AMINE |
Computed Descriptors
| IUPAC Name | (NE)-N-[3-[(2-chloro-1,3-thiazol-5-yl)methyl]-5-methyl-1,3,5-oxadiazinan-4-ylidene]nitramide |
| SMILES | CN\1COCN(/C1=N/[N+](=O)[O-])CC2=CN=C(S2)Cl |
| Canonical SMILES | CN1COCN(C1=N[N+](=O)[O-])CC2=CN=C(S2)Cl |
| InChI | InChI=1S/C8H10ClN5O3S/c1-12-4-17-5-13(8(12)11-14(15)16)3-6-2-10-7(9)18-6/h2H,3-5H2,1H3/b11-8+ |
| InChIKey | NWWZPOKUUAIXIW-DHZHZOJOSA-N |
Computed Properties
Show more
| Molecular Weight | 291.72 g/mol |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 291.019 |
| Monoisotopic Mass | 291.019 |
| Topological Polar Surface Area | 115 |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 352 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
Annotation
31 pairs of Verified P450 and Thiamethoxam
| Insect | Gene Symbol | Validation | Reference |
|---|---|---|---|
| Aphis gossypii | CYP4CK1 | R,D | |
| Aphis gossypii | CYP6CY13 | X | |
| Aphis gossypii | CYP6CY14 | R | |
| Aphis gossypii | CYP6CY22 | X | |
| Aphis gossypii | CYP6CY9 | D | |
| Aphis gossypii | CYP6CZ1 | R,D | |
| Aphis gossypii | CYP6DA1 | R,X | |
| Aphis gossypii | CYP6DB1 | R,D | |
| Aphis gossypii | CYP6CY9 | R,D | |
| Bemisia tabaci | CYP4CS5 | R,D,X | |
| Bemisia tabaci | CYP4G68 | R | |
| Bemisia tabaci | CYP6CM1 | R,E | |
| Bemisia tabaci | CYP6CX2 | R | |
| Bemisia tabaci | CYP6CX3 | R | |
| Bemisia tabaci | CYP6DB3 | R,D,X | |
| Bemisia tabaci | CYP6DV5 | R | |
| Bradysia odoriphaga | CYP3356A1 | R | |
| Bradysia odoriphaga | CYP3A56 | R | |
| Bradysia odoriphaga | CYP6FV2 | R,E | |
| Bradysia odoriphaga | CYP9J35 | R | |
| Drosophila melanogaster | CYP6G1 | X | |
| Leptinotarsa decemlineata | CYP9AY1 | R | |
| Leptinotarsa decemlineata | CYP9Z140 | R | |
| Myzus persicae | CYP6CY3 | R | |
| Nilaparvata lugens | CYP6CS1 | R | |
| Nilaparvata lugens | CYP6ER1 | R,D | |
| Sitobion miscanthi | CYP307A2 | R | |
| Sitobion miscanthi | CYP4CJ6 | R | |
| Sitobion miscanthi | CYP6DD1 | R | |
| Spodoptera frugiperda | CYP340AD3 | R | |
| Spodoptera frugiperda | CYP4G74 | R |