Spinosyn A
| PubChem CID | 443059 |
| Molecular Formula | C41H65NO10 |
| Structure |
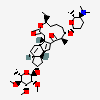
|
| Synonyms |
Spinosyn A
131929-60-7 Spinosad factor A Lepicidin A A 83543A CHEBI:9230 OY0L59V61N (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-[(6-Deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy]-13-[[(2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl]oxy]-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-1H-as-indaceno[3,2-d]oxacyclododecin-7,15-dione Spinosyn-A Spinosad A (1S,2R,5S,7R,9R,10S,14R,15S,19S)-15-[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy-19-ethyl-14-methyl-7-[(2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyloxan-2-yl]oxy-20-oxatetracyclo[10.10.0.02,10.05,9]docosa-3,11-diene-13,21-dione (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]oxy}-9-ethyl-14-methyl-7,15-dioxo-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-1H-as-indaceno[3,2-d]oxacyclododecin-2-yl 6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranoside 2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-(6-deoxy-2,3,4-tri-O-methyl-?-L-mannopyranosyloxy)-13-(4-dimethylamino-2,3,4,6-tetradeoxy-?-D-erythropyranosyloxy)-9-ethyl-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-14-methyl-1H-as-indaceno[3,2-d]oxacyclododecine-7,15-dione (-)-Spinosyn A Spinosad factor A [USAN] UNII-OY0L59V61N CCRIS 8937 HSDB 7006 SPINOSYNS [MI] SCHEMBL465876 CHEMBL501411 DTXSID8037598 SPINOSAD FACTOR A [HSDB] HY-B0767 MFCD28388854 AKOS032946512 1ST20443 DA-67721 CS-0009656 NS00004555 G14465 Q27108323 (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-2-((6-Deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy)-13-(((2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl)oxy)-9-ethyl-14-methyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-1H-as-indaceno(3,2-d)oxacyclododecin-7,15-dione 1H-as-Indaceno(3,2-d)oxacyclododecin-7,15-dione, 2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-2-((6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy)- 13-((5-dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl)oxy)-9-ethyl-14-methyl-, (2R-(2R*,3aS*,5aR*,5bS*,9S*,13S*(2R*,5S*,6R*),14R*,16aS*,16bR*))- 1H-as-Indaceno(3,2-d)oxacyclododecin-7,15-dione, 2-((6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranosyl)oxy)-13-(((2R,5S,6R)-5-(dimethylamino)tetrahydro-6-methyl-2H-pyran-2-yl)oxy)-9-ethyl-2,3,3a,5a,5b,6,9,10,11,12,13,14,16a,16b-tetradecahydro-14-methyl-, (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)- |
Computed Descriptors
| IUPAC Name | (1S,2R,5S,7R,9R,10S,14R,15S,19S)-15-[(2R,5S,6R)-5-(dimethylamino)-6-methyloxan-2-yl]oxy-19-ethyl-14-methyl-7-[(2R,3R,4R,5S,6S)-3,4,5-trimethoxy-6-methyloxan-2-yl]oxy-20-oxatetracyclo[10.10.0.02,10.05,9]docosa-3,11-diene-13,21-dione |
| SMILES | CC[C@H]1CCC[C@@H]([C@H](C(=O)C2=C[C@H]3[C@@H]4C[C@@H](C[C@H]4C=C[C@H]3[C@@H]2CC(=O)O1)O[C@H]5[C@@H]([C@@H]([C@H]([C@@H](O5)C)OC)OC)OC)C)O[C@H]6CC[C@@H]([C@H](O6)C)N(C)C |
| Canonical SMILES | CCC1CCCC(C(C(=O)C2=CC3C4CC(CC4C=CC3C2CC(=O)O1)OC5C(C(C(C(O5)C)OC)OC)OC)C)OC6CCC(C(O6)C)N(C)C |
| InChI | InChI=1S/C41H65NO10/c1-10-26-12-11-13-34(52-36-17-16-33(42(5)6)23(3)48-36)22(2)37(44)32-20-30-28(31(32)21-35(43)50-26)15-14-25-18-27(19-29(25)30)51-41-40(47-9)39(46-8)38(45-7)24(4)49-41/h14-15,20,22-31,33-34,36,38-41H,10-13,16-19,21H2,1-9H3/t22-,23-,24+,25-,26+,27-,28-,29-,30-,31+,33+,34+,36+,38+,39-,40-,41+/m1/s1 |
| InChIKey | SRJQTHAZUNRMPR-UYQKXTDMSA-N |
Computed Properties
Show more
| Molecular Weight | 732 g/mol |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 731.461 |
| Monoisotopic Mass | 731.461 |
| Topological Polar Surface Area | 111 |
| Heavy Atom Count | 52 |
| Formal Charge | 0 |
| Complexity | 1290 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 17 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
4 pairs of Verified P450 and Spinosyn A
| Insect | Gene Symbol | Validation | Reference |
|---|---|---|---|
| Frankliniella occidentalis | CYP6A2 | R | |
| Leptinotarsa decemlineata | CYP4G15 | R | |
| Leptinotarsa decemlineata | CYP6A23 | R | |
| Leptinotarsa decemlineata | CYP9E2 | R |